Atoms or groups attached to an allylic carbon are termed allylic substituents.
Allylic and vinylic hydrogens.
An allylic carbocation in which an allylic carbon bears the positive charge.
Allylic vinylic examples organic chemistry duration.
The libretexts libraries are powered by mindtouch and are supported by the department of education open textbook pilot project the uc davis office of the provost the uc davis library the california state university affordable learning solutions program and merlot.
Chlorination of allylic hydrogen is difficult than vinylic hydrogen.
The key difference between allylic and vinylic carbon is that allylic carbon is the carbon.
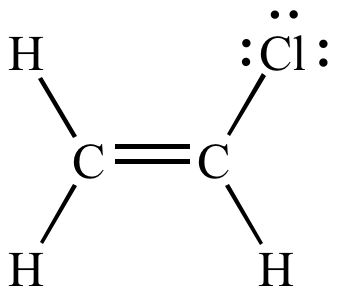
Identify the number of allylic and vinylic hydrogens in the pictured molecules.
An allylic hydrogen is a hydrogen atom that is bonded to an allylic carbon in an organic molecule.
Allyl group holds three carbon atoms and five hydrogen atoms on the other hand vinyl group has two carbon atoms and three hydrogen atoms.
A hydrogen atom bonded to an sp 2 carbon of an alkene.
Allyl form a stable carbocation because of the electron delocalization whereas vinylic carbocations are unstable as they lack p character.
Benzylic position allylic position propargylic position aryl aryl hydrogen.
In other words it is a methylene bridge ch 2 attached to a vinyl group ch ch 2.
Key difference allylic vs vinylic carbons functional groups are very important in understanding the different physical and chemical properties of organic molecules the terms allylic and vinyl carbons indicate whether the carbon atom is bonded directly or indirectly to a double bond in a molecule.
An allylic carbon is an sp3 carbon that is adjacent to a vinylic carbon.
When one hydrogen atom is removed from the third carbon atom of a propane molecule it is equivalent to an allyl group.
The vinylic hydrogens are shown in red.
Br allyl radical is stabilished by resonance.
We also acknowledge previous national science foundation support under grant numbers 1246120 1525057 and 1413739.
It contains two sp 2 hybridized carbon atoms and one sp 3 hybridized carbon atom.